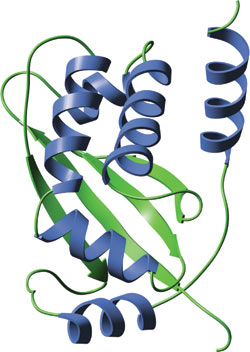
The monomeric TRAF domain of human TRAF2.
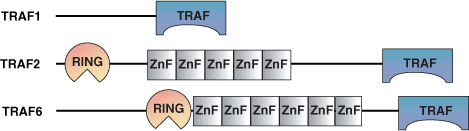
The approximately 150 amino acid TRAF domain is found in Tumor Necrosis Factor (TNF) receptor-associated factors. TRAF proteins appear to be a relatively recent evolutionary development as there is just one C. elegans TRAF protein and only two Drosophila, and six mammalian TRAF proteins. All mammalian TRAFs localize to the cytoplasm except TRAF4 which is found in the nucleus. TRAF proteins are recruited to the membrane through interactions of their TRAF domains with activated TNF receptors, IL-1/Toll receptors or through intermediate proteins such as the TRADDs. TRAFs primarily act in cell survival upon interacting with TNF receptors by activating the NF- κB and AP-1 transcription factors. The six mammalian TRAF proteins have distinct functions. For example, TRAF3 regulates T-cell dependent antigen responses, TRAF4 is required for formation of the trachea and TRAF6 modulates IL-1, CD40, and LPS signaling. TRAFs are also important in Epstein-Barr Virus replication by binding to LMP1 and subsequently potentiating growth and transformation.
TRAF domains are composed of a highly conserved coiled-coil domain followed by a TRAF-C domain. The N-terminal coiled-coil domain is comprised of a single α helix while the TRAF-C domain folds into a β-sandwich structure. TRAF domains oligomerize as dimers, trimers and/or heterotrimers. The trimeric structure of the TRAF domain of TRAF-2 is a coiled-coil region that forms a single stalk-like structure with TRAF-C domains forming three separate lobed structures below this stalk.
| TRAF Domain Proteins | Binding Partners |
| TRAF 1,2,3,5 | CD40 |
| TRAF 1,2 | TRADD |
| TRAF6 | IRAK |
| TRAF 2 | TNFR1 |
| TRAF 6 | IL-1 |