Our extensive validation and advanced technical support ensures that all of our IHC-P-validated antibodies can be used for fluorescent multiplex IHC.
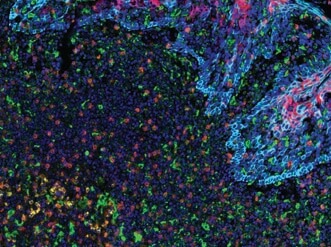
PD-L2 (orange), PD-L1 (E1L3N®) (red), CD68 (green), PD-1 (yellow), CD8α (magenta) and Pan-Keratin (cyan) on FFPE tonsil.
Being able to visualize multiple targets simultaneously is important in research fields like tumor immunology where it is necessary to catalog subsets of immune and cancer cells within the tumor microenvironment. For example, tumors may evade immune detection by manipulating the expression of immune checkpoint proteins (e.g., PD-1, CTLA-4, TIM-3) to “turn off” activated T cells. Alternatively, T cells may become less effective at destroying tumor cells as they succumb to exhaustion, a phenomenon characterized by changes in immune checkpoint protein expression, T cell expression of VISTA, and the appearance of CD68+ co-infiltrating macrophages. Thus, molecular profiling of immune checkpoint proteins together with immune cell phenotyping is key to understanding the complex tumor microenvironment and to enabling the development of tailored, combinatorial therapeutic intervention.
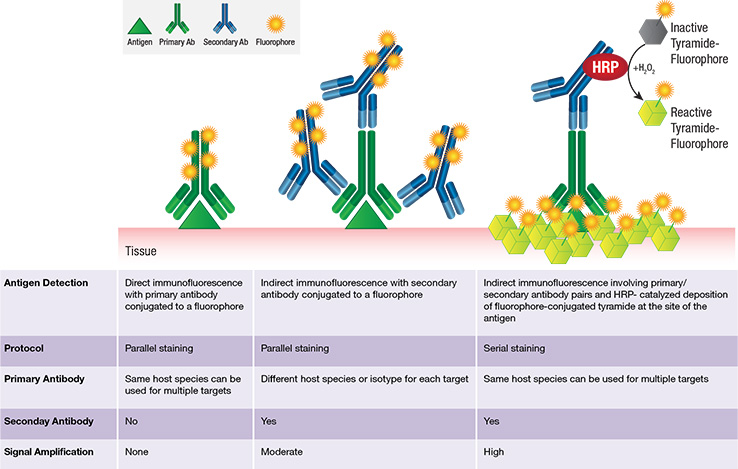
Tyramide is an organic phenol that, in the presence of a catalyst (HRP), becomes activated and covalently binds to electron rich regions, typically tyrosine residues, present on the surface or in the vicinity of protein antigens (see schematic below). Thus HRP-catalyzed deposition of tyramide-fluorophore molecules provides enhanced amplification of fluorescence signal at the site of the antigen.
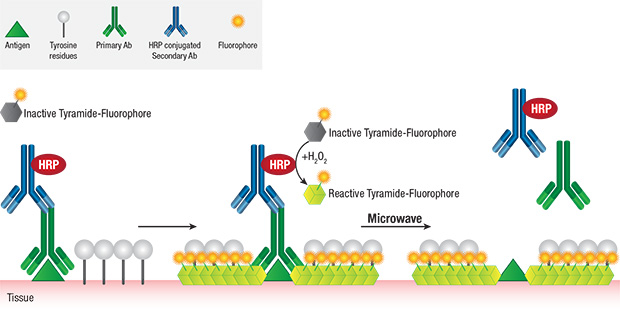
The covalent nature of tyramide-tyrosine engagement allows for heat-mediated removal (stripping) of primary/secondary antibody pairs bound to the antigen, while preserving the antigen-associated fluorescence signal. This facilitates the sequential use of multiple primary antibodies of the same host species or isotype without the concern for crosstalk, thereby greatly enabling multiplexing potential.
Tyramide-based Fluorescent mIHC requires the following factors:
The size of the antibody panel largely determines the imaging system of choice.
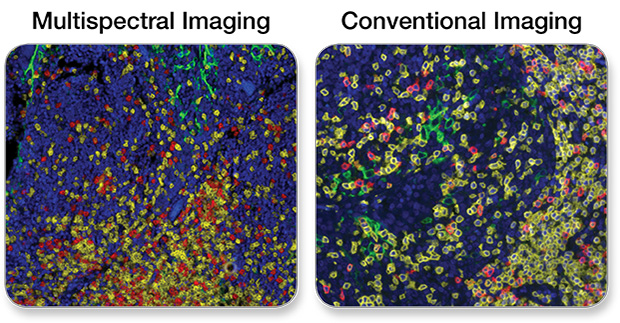
PD-L1, CD3ε, CD8α Multiplex IHC Panel #65713: A 3-plex Fluorescent mIHC analysis of paraffin-embedded human breast cancer using PD-L1 (E1L3N®) XP® Rabbit mAb #13684 (green), CD3ε (D7A6E) XP® Rabbit mAb #85061 (yellow), and CD8α (C8/144B) Mouse mAb (IHC Specific) #70306 (Red). Blue pseudocolor = DAPI #8961 (fluorescent DNA dye).
Note: Tissue autofluorescence can be an issue often contributing to poor sensitivity in fluorescent IHC. This phenomenon is apparent in the context of all fluorescent imaging platforms. Multispectral analysis software addresses this issue by subtracting the artifactual fluorescence signal that originates from unstained tissue.